Some Important Compounds of Transition Metals
Some Important Compounds of Transition Metals: Overview
This topic covers concepts, such as, Important Compounds of d-Block Elements, Potassium Dichromate, Oxidation of Manganese (II) Ion to Manganese Dioxide & Uses of Potassium Permanganate etc.
Important Questions on Some Important Compounds of Transition Metals
A compound which is a strong oxidizing agent and has orange coloured crystal. It is used in the preparation of azo compounds. Identify the compound:
On oxidation of by in neutral aqueous medium, the oxidation number of S would change from:
On complete oxidation of in acidic medium, the oxidation state of Cr will change from:
The solution of potassium dichromate is prepared from which of the following compound and how its colour changes with change in pH:
The number of moles of that will be needed to react with one mole of sulphite ion in acidic solution is:
In alkaline medium, the reduction of permanganate anion involves a gain of ____________ electrons.
In the extraction process of copper, the product obtained after carrying out the reactions
is called
Given below are two statements:
Statement I : Aqueous solution of is preferred as a primary standard in volumetric analysis over aqueous solution.
Statement II : has a higher solubility in water than . In the light of the above statements, choose the correct answer from the options given below:
During the reaction of permanganate with thiosulphate, the change in oxidation of manganese occurs by value of . Identify which of the below medium will favour the reaction.
Ferric chloride is applied to stop bleeding because
How many electrons are gained by in strongly alkaline medium.
During bleeding from a cut is used to stop bleeding as:
Read the following two statements.
Statement I: Potassium dichromate is used in volumetric analysis.
Statement II: is more soluble in water than
Oxidation state of in changes by three units in which medium ?
Explain oxidising nature of Potassium Permanganate in acidic medium?
The following steps are involved in the manufacturing of potassium dichromate:
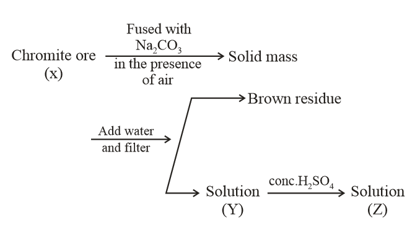
What is the difference in the oxidation number of between X and Y?
Which of the following reaction is spontaneous oxidation-reduction reaction?
When crystals are heated with conc. , the gas evolved is
In the dichromate ion
Which of the following species has oxygen in oxidation state?
